This is a short story about how life came about on this planet and probably in this universe. it is not a story of a supreme being or creation but an even more difficult to understand and believe story of the Big Bang and what has happened since. This is not an exhaustive story but a brief sketch for the layman who wants to know how he got here in a relatively simple manner without difficult scientific explanations and without resort to witchcraft.
Those of a religious bent will find little to dispute with this story and might take small comfort in that it does not explain what came before the Big Bang or what lies outside or after this universe. But those matters are hardly likely to worry the human race whose part in this unfolding drama is likely to be very brief indeed.
The big Bang occurred 13.75 billion years ago when a singularity - ie a very small point in space - decided to expand. We know with some certainty only those things that have happened since after the first billionth of a second or so. Before that we cannot yet go. After that the universe consisted of a substance whose nearest description we now call plasma. This is a very hot - ie everything is moving at or near the speed of light - mixture of energy quantums. As this cooled the particles we know separated out from it to become protons and electrons. This process took about three minutes by our time standards but before this time as we know it had no meaning.
After about one hundred thousands years the particles had settled into matter as we know it. There were protons and neutrons and electrons and weird stuff like neutrinos. The whole lot then began to cool quite rapidly.
The particles began to hook together by atomic attraction and some of the energy was thereby turned into mass to give us the mix that we still have today. Most of these particles formed hydrogen which is the simplest of all atoms and those proliferated along with significant amounts of helium. The gravity created by the mass of the particles caused random scatterings of particles to coalesce into clouds which generally started to rotate and further coalesce.
When the accretion of particles reached a suitable size it began to compress under the effects of its own gravity and squeezed inwards until the central density and temperature reached a critical value and a new sun started what we now call a nuclear reaction to occur at its centre.
Many of these had huge amounts of material and formed very large stars which burned up their available hydrogen quite fast. The star burned hydrogen to form helium and then went through numerous different processes which created all the other elements up as far as iron. Eventually the star would experience several stages which finished with one or more explosions. These could - and probably did - produce all the other elements up to Uranium. When the star finally exploded its constituent molecules would be spread through the empty spaces to help create the next generation of stars. it is likely that there have been many generations of the big hot, rapidly progressing stars.
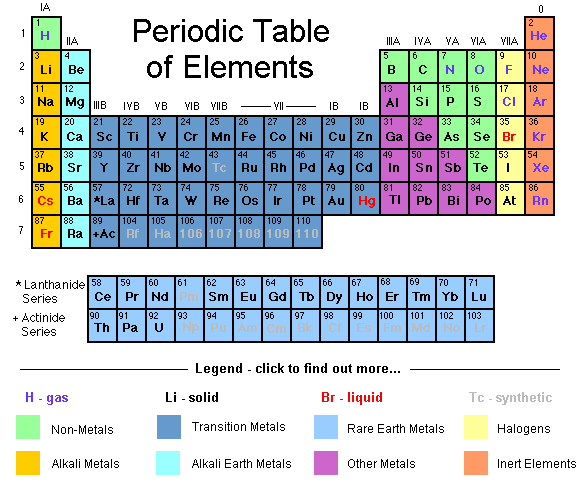
The elements consist of protons, electrons and neutrons. The neutron has no electric charge and each positively charged proton attracts a negatively charged electron. The simplest atom constructed in this way is hydrogen which has just one proton and one electron.
Hydrogen Atom
The periodic table above shows all the elements with each one having one more proton and electron than the previous element. Every possible element has been found on earth up to Uranium with 92 protons - although some in very minute quantities.
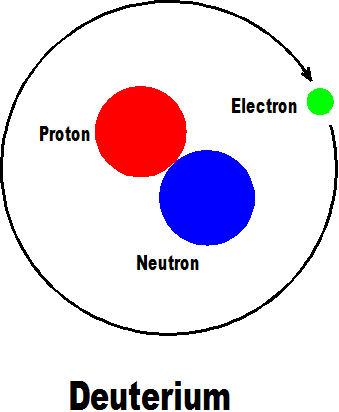
The next element is deuterium with has an added neutron but still only one proton so is strictly an isotope of hydrogen.
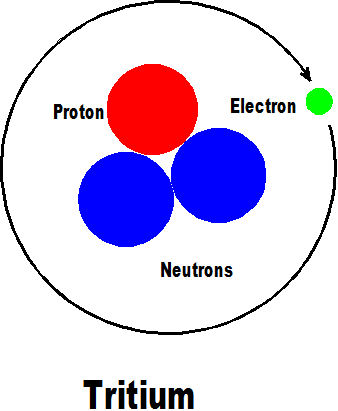
The next element is tritium which has two added neutrons but still only one proton and one electron and is another isotope of hydrogen.
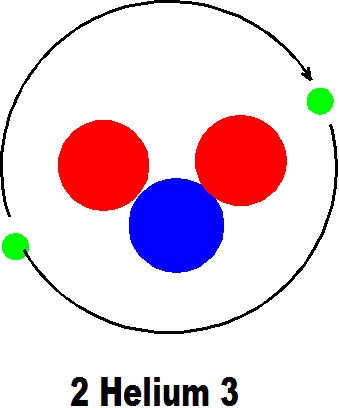
2 Helium 3 is the next truly different element because it has another proton and another electron which makes it totally different to hydrogen. All atoms occupy more or less the same space so that these elements with few protons have a very low weight so that hydrogen and helium are the lightest elements of all.
The first number in the name here shows the number of protons and the second the total number of protons and neutrons. The number of electrons is usually the same as the number of protons.
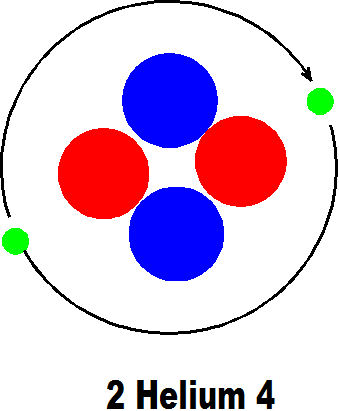
2 Helium 4 is another isotope of Helium but still has two protons and two electrons. In principle the atoms of every element have the same number of protons as electrons. The positive charge on the proton attracts a negatively charged electron to make the atom neutral overall. There are exceptions to this rule but they usually exist for only short periods of time.
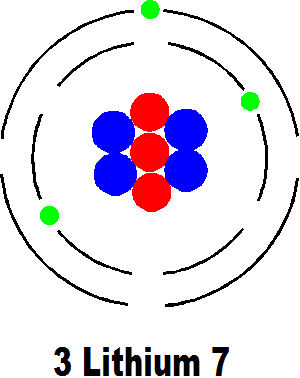
The next element is lithium with three protons and electrons. You can see how four neutrons are conveniently attracted into this scheme. But the atom is nowhere near as simple as this. The drawings here are convenient ways of simply depicting atoms and are surprisingly useful for many purposes but an actual atom looks nothing like this at all.
The better picture of the atom would be a model of the solar system with the sun at the middle and the planets revolving round it. In a similar way the protons and neutrons (collectively called hadrons) gather at the core and the electrons revolve rapidly around them. The atom, like the solar system, is mostly empty space. But unlike the solar system, where the planets continue on their stately journeys in very predictable circles and ellipses for billions of years, the electrons, could we see them, would look like a ball of wire wool.
Indeed there is a notion called the Uncertainty Principle that says that it is impossible to define both the position and velocity of an electron at any given time. Some people have tried to extend this basic uncertainty to claim that nothing can be certain but the majority of atoms continue their merry way in an absolutely predictable manner for billions of years. The extension of the uncertainty principle to real life is not realistic.
Nature then continued the addition of a proton to create beryllium and then boron which are very useful substances but not in the ken of most people on earth and they are not important to our story of life on earth but play an important part in the sun.
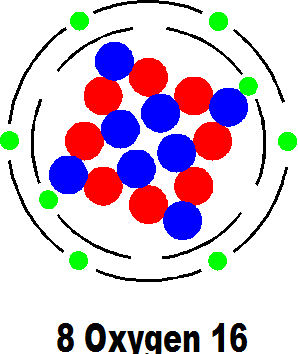
Then nature created carbon and this is the basis of all we know as life and energy. We shall see how it does this later. There is, incidentally, a very useful isotope of carbon called 6 carbon 14 which allows the very accurate dating of archeological remains.
Early life quite possibly consisted of only molecules of carbon and hydrogen and they synthesised nitrogen and oxygen. Gradually the atmosphere of the earth began to accumulate oxygen and the basic organisms had to adapt to exist alongside it. Eventually the agglomeration of atoms into molecules occurred, probably quite by accident and after countless billions of trials, into the molecules we now know as life. It is quite possible that at this stage the planet was seeded by molecules from outer space; but then, the planet was completely constructed with material from outer space.
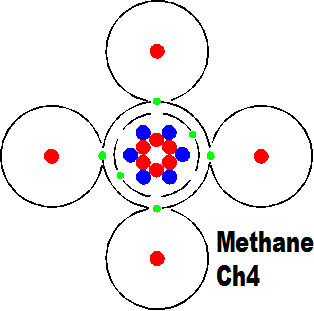
Another significant event was the banding together of carbon and hydrogen to form molecules that are the basis of energy to this day. This is the most basic molecule of energy storage. you can see how a single carbon atom can attract and carry four atoms of hydrogen. The carbon is just a carrier and the fuel is the hydrogen. The methane molecule is the simplest to carry energy.
Butene is another fairly simple molecule and the basic construction is similar. All the molecules of fuel such as oil, paraffin, gas and even coal carry this basic structure. Some of the molecules are huge and become almost unintelligible (to me). But all store energy.
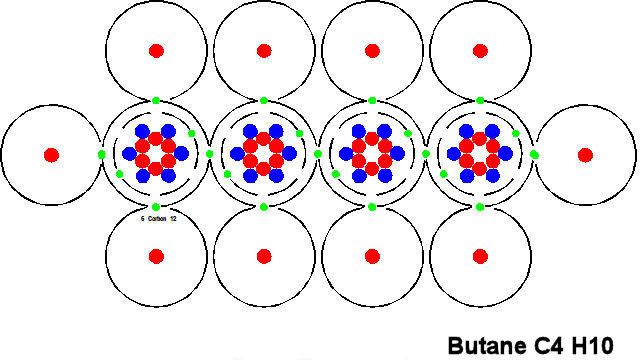
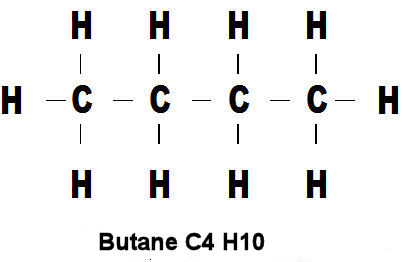
If you now look at the picture below you will see that is is identical to the one above except that we have replaced the little pictures of atoms with single letters. We shall adopt this custom (mostly) from now on as it is easier to remember and understand.
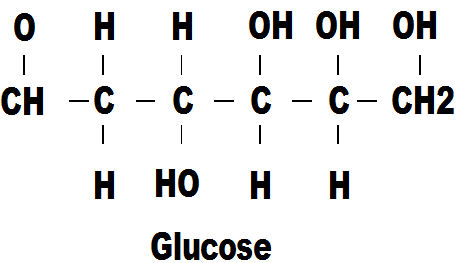
Now we come to the first molecule to incorporate oxygen. You can see that its basic layout is similar to that of butane but with a few more carbon atoms and several oxygen atoms in various combinations. Glucose is a sugar and is a source of energy for life forms and it is no accident that it is so similar to the energy form butane. The oxygen makes it compatible with, and usable by, life forms. So this is how we get the energy to maintain our lives. There are many variations of glucose and other sugar molecules.
Two other molecules we must show to complete the circus of actors in this story of life. The first is co2 which acts like a dustbin to take away the unwanted carbon after the hydrogen has surrendered its energy. it is invariable a waste product in the life of animals and is recycled by plants and oceans and the atmosphere in chemical reactions which are often extremely complicated.
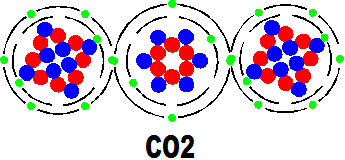
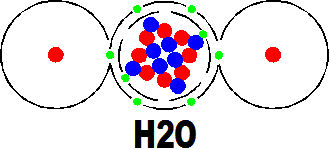
The other artist in our story is water (H2o) which is a quite simple molecule but by far the most important secondary molecule in the story of life.
So now we come to the life molecules. The first is adenine. you can see it contains only carbon and hydrogen, with which we are familiar, together with some nitrogen in various combinations. This is the first of the "genes" which molecules are the basis of the DNA molecule which is the basis of the living cell.
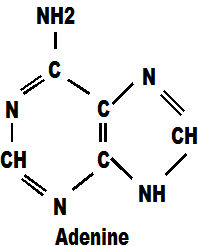
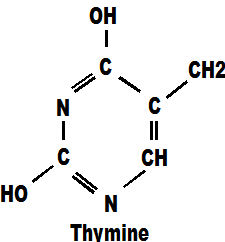
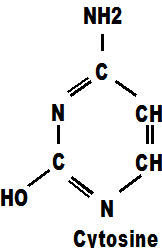
Adenine always pairs with thymine in the DNA chain and it too, contains mainly carbon and hydrogen atoms mixed with some oxygen and nitrogen. within the DNA molecule these molecules are referred to as 'a' and 't'.
The second pair of molecules, called 'g' and 'c' are firstly guanine which has only carbon, hydrogen and nitrogen.
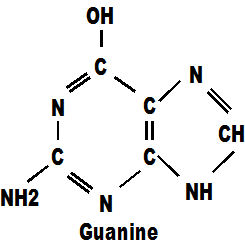
Cytosine is similar but has the addition of an oxygen atom. These four molecules between them, with only four types of atoms, make up the whole of our DNA chains. Those chains carry every secret of life from one generation to the next. They tell a foetus how to develop, they tell a tree how to grow, they reproduce themselves endlessly until the moment of death and they create the myriad multitude of cells necessary to satisfy the highly varied and demanding requirements of different parts of the body. They do this for every single life form on the planet above the size of virus. There are only four genes and they appear together in every single life-form ever known on earth. The genes a,c,g,t are a world class act.
And that is the basis of all life as we know it, Jim.
But there is one more story to tell. The whole of life depends upon this hydro-carbon energy cell. There is a fundamental rule of physics that all energy "runs down". This is known as the Law of Entropy. You cannot simply create energy, you have to get it from somewhere and usually to intercept it on the way from one place to another. So where does our energy come from?
We know from experience our energy comes from the sun. But where does the sun get it from? The answer is that there are isolated, and temporary, exceptions to the decay of entropy. These are called stars and our sun is simply a typical star.
The Big Bang created hydrogen and helium in the ratio of around 66 to 34 parts. When these two elements compress together into a star they heat up under the pressure of gravity and start a nuclear interaction. The interaction essentially turns hydrogen into helium and when the hydrogen is used up the star will die. The story is much more complicated than that but this is a primer, not an exposition of nuclear physics.
The basic reaction at the core of the the sun is shown below. Remember this is all very very hot and these particles are moving at very high speeds. Two protons bash together to form deuterium and give off a neutrino and a positron which gets hit by an electron and disintegrates to give off two photons of light.
The deuterium atom then gets bashed by another proton which creates helium 3 and gives off another photon. This is the basic reaction.
Below I have shown the basic reaction three times. Two molecules of helium 3 then bash together, lose two protons and form helium 4. This new molecule then gets bashed by another molecule of helium 3 which creates a molecule of beryllium 7. This then gets bashed by another proton and gives off another photon while forming boron. The boron transmutes to beryllium 8. The beryllium disintegrates into two molecules of helium. This reation gives off a positron which takes an electron to give off another couple of photons and a neutrino is also released. Thus the original protons (hydrogen) are transmuted into helium.
Conversion of Hydrogen to Helium in a star.
The interesting thing to note is that as well as giving off 12 photons this process has also given off 4 neutrinos. We see the photons of light all around us as energy from the sun. There are billions of them. There are also billions of neutrinos but we have yet to detect more than a handful. They are so light that they can go right thorough the earth without even noticing and it is very difficult to find any way to stop or detect them. This is one of the continuing problems of science.
The above is a very basic transformation and there are many more reactions going on in a star which may change with time and the size of star. By similar means a star can make all elements up to and including 26 Iron. The higher elements are formed in the spectacular death throes of stars and usually thrown out across space.
Our sun is about eight billion years old and is likely to remain hospitable for another three billion years. The universe is about 13.7 billion years old and its future has not yet been ascertained.
So that is the story of where we came from and where we are and what we are and with little idea of where we are going. But it's quite a story.